During the recent national congress of SIN (Italian Society of Nephrology) Prof. Grandaliano presented interesting concepts on the role of the dialysis membrane in CKD patients, in particular patients who are waitlisted for transplantation. In addition, promising results on the use of PMMA dialysis membrane in COVID-19 patients were presented.
The CKD-patient waitlisted for transplantation: immunomodulation and role of the dialysis membrane
Immunological features of hemodialysis patients
The length of pre-transplant dialysis is associated with premature graft loss and mortality after kidney transplantation (1). When patients enter in hemodialysis their immune-pattern changes compared to un-dialyzed CKD patients and alter the expression of genes involved in the immune response (2). Patients undergoing maintenance hemodialysis have impaired immune responsiveness, which appears to deteriorate progressively with the duration of the dialysis treatment (3). The hemo-compatibility and the bio-compatibility of the dialysis membrane is fundamental in this aspect. Blood-membrane contact affects both innate and adaptive immunity (3). T cells are the major mediators of adaptive immunity and have been shown to be altered according to dialysis modality (4). The balance between regulatory T cells (Treg) and T helper (Th)17 cells play an important role in the development of inflammatory and autoimmune diseases. The Treg/Th17 balance is disturbed by uremia, especially in patients with adverse cardiovascular events. This Th17/Treg imbalance might act synergistically with microinflammation on immune-mediated atherosclerosis and contribute to the high incidence of adverse cardiovascular events (5, 6).
Delayed graft function and allograft survival
DGF depends on the donor type and its incidence may vary from 10 to 40%. DGF negatively influences graft survival. Patients who experience DGF have a lower number of Treg at transplantation compared to those who gain full kidney functionality (7). Patients with DGF show a significant increase of circulating Th17 cells after transplantation (8). DGF is accentuated in patients who present interstitial graft infiltration of Th17 cells (9). IL-6 plasma levels are associated with circulating Th17 and Treg cell numbers (5). A major cause of DGF is ischemia-reperfusion damage generated by activation of complement system and by coagulation pattern (10).
PMMA – from inactivation of Complement to 3-dimensional hemodialysis
The dialyzer membrane in polymethylmethacrylate (PMMA) in addition to its outstanding hemo and biocompatibility (no activation of the complement system, no activation of inflammatory-coagulation host patterns) adds a third dimension to dialysis: adsorption. Whereas diffusion and convection remove small and medium sized molecules, adsorption has the capacity to remove medium and high molecular weight molecules responsible for many complications in the uremic patient. Membrane biocompatibility is an important aspect and PMMA has been shown to reduce circulating levels of IL-6 (11, 12).
Forty percent of patients awaiting transplantation have circulating anti-HLA antibodies, increasing the likelihood of antibody-mediated rejection, the main cause of graft loss. Patients with antibody-mediated rejection have an increased gene expression of proteins involved in the IF- signaling pathway (13). Blood-membrane contact induces an overproduction of IF-alpha (14). PMMA has been shown to be effective in its removal (15), thus allowing to intervene on the mechanisms underlying the antibody-mediated rejection. The concentration of the MxA protein, whose function is strictly dependent on the IF- concentration, is significantly reduced when passing from a PS membrane to a PMMA membrane.
PMMA – adding a fourth dimension to dialysis
The new generation of PMMA membranes also represent a fourth dimension to dialysis: antithrombogenicity. Dialysis nearly always produce a deposit of platelets and fibrin along the fibers due to the activation of the coagulation cascade (16). Coagulation factors intervene in filter or vessel clotting and are able to interact with specific membrane receptors and thereby modulating the immune response. The new modified PMMA membrane, Filtryzer® NF, is able to minimize platelet and fibrinogen adhesion on the filter surface and reduces the release of thromboglobulin produced by the platelets once the thrombus is formed (17).
The PMMA membrane is known for its marked hemocompatibility. Several studies have shown that dialysis conducted with a membrane PMMA reduces the expression of complement factors, C3a and C5a, and mediators of the inflammatory response such as IL-6, IL-8, TNF-α and MCP-1 (11).
It has been shown that attenuating coagulation activation during the dialysis session using special filters, circulating lymphomonocytes reduce the expression of CCR-2, receptor for MCP-1, a chemotactic protein for monocytes (18).
Before transplantation, patients who develop DGF have a high concentration of CCR-2 on circulating lymphomonocytes. Among other things, the graft, especially when it comes from a brain-dead donor, is characterized by a large production of MCP-1 (19).
High levels of MCP-1 released by the kidney result in increased expression of CCR-2, resulting in greater recruitment of inflammatory cells. A membrane that reduces the activation of coagulation therefore allows this phenomenon to be attenuated.
COVID-19 and the role of the dialysis membrane
Some of the most significant clinical manifestations of SARS-CoV-2 are related to the activation of complement system, activation of inflammatory cascade with a dysregulated host response that can lead in some cases to the release of relevant amounts of cytokines and other inflammatory mediators. Using a membrane that is able to minimize the activation of complement system and pro-inflammatory response and in addition removes cytokines during hemodialysis treatment can be an effective strategy. Data from patients (n = 24) treated in four different hospitals in Italy (PMMA vs PS hemodialysis groups were retrospectively analyzed. These preliminary results show that patients treated with PMMA had a markedly higher survival rate than patients who received PS hemodialysis (see figure below).
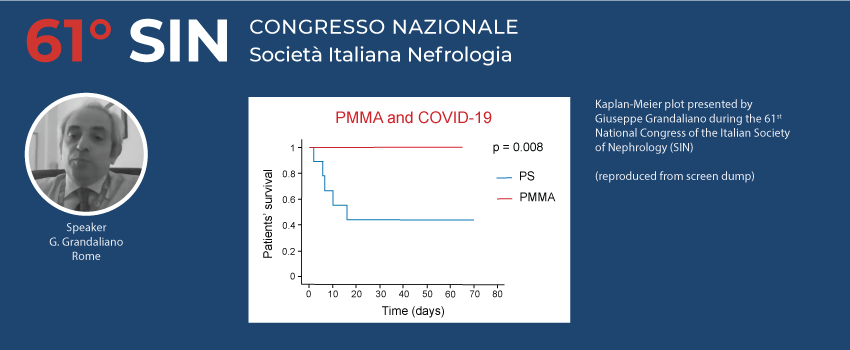
In view of the arrival of the vaccine for SARS-CoV-2, it is important to emphasize the importance of the vaccine response. Patients on hemodialysis have a markedly reduced vaccination response due to their chronic immune-compromised pattern. Previously, it has been shown that the use of PMMA in patients not responding to the hepatitis B vaccine significantly increased their response (20), in turn linked to the reduction in blood concentration of sCD40, a protein implicated in the vaccine response, effectively removed by PMMA (21). Protecting hemodialysed patients, to guarantee a better response to seasonal anti-flu vaccine, should be a clinical target in general, and above all, in the COVID-19 era.
These effects of the PMMA membrane on the immunological system of hemodialysis patients may explain the results of studies showing a reduction in mortality compared to other membranes (22).
The webinar (in Italian) can be watched on-demand by registered participants on the congress website.
References
- Haller MC, Kainz A, Baer H, Oberbauer R. Dialysis Vintage and Outcomes after Kidney Transplantation: A Retrospective Cohort Study. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(1):122-30.
- Zaza G, Granata S, Rascio F, Pontrelli P, Dell’Oglio MP, Cox SN, et al. A specific immune transcriptomic profile discriminates chronic kidney disease patients in predialysis from hemodialyzed patients. BMC Med Genomics. 2013b;6:17.
- Donati D, Degiannis D, Combates N, Raskova J, Raska K, Jr. Effects of hemodialysis on activation of lymphocytes: analysis by an in vitro dialysis model. Journal of the American Society of Nephrology : JASN. 1992;2(10):1490-7.
- Xiaoyan J, Rongyi C, Xuesen C, Jianzhou Z, Jun J, Xiaoqiang D, et al. The difference of T cell phenotypes in end stage renal disease patients under different dialysis modality. BMC nephrology. 2019;20(1):301.
- Zhang J, Hua G, Zhang X, Tong R, Du X, Li Z. Regulatory T cells/T-helper cell 17 functional imbalance in uraemic patients on maintenance haemodialysis: a pivotal link between microinflammation and adverse cardiovascular events. Nephrology (Carlton, Vic). 2010;15(1):33-41.
- Afzali B, Edozie FC, Fazekasova H, Scottà C, Mitchell PJ, Canavan JB, et al. Comparison of regulatory T cells in hemodialysis patients and healthy controls: implications for cell therapy in transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(8):1396-405.
- Nguyen MT, Fryml E, Sahakian SK, Liu S, Cantarovich M, Lipman M, et al. Pretransplant Recipient Circulating CD4+CD127lo/- Tumor Necrosis Factor Receptor 2+ Regulatory T Cells: A Surrogate of Regulatory T Cell-Suppressive Function and Predictor of Delayed and Slow Graft Function After Kidney Transplantation. Transplantation. 2016;100(2):314-24.
- Loverre A, Divella C, Castellano G, Tataranni T, Zaza G, Rossini M, et al. T helper 1, 2 and 17 cell subsets in renal transplant patients with delayed graft function. Transpl Int. 2011;24(3):233-42.
- Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. Journal of immunology (Baltimore, Md : 1950). 2010;184(9):5344-51.
- Pontrelli P, Cariello M, Rascio F, Gigante M, Verrienti R, Tataranni T, et al. Thrombin may modulate dendritic cell activation in kidney transplant recipients with delayed graft function. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association. 2015;30(9):1480-7.
- Pertosa G, Grandaliano G, Gesualdo L, Ranieri E, Monno R, Schena FP. Interleukin-6, interleukin-8 and monocyte chemotactic peptide-1 gene expression and protein synthesis are independently modulated by hemodialysis membranes. Kidney international. 1998;54(2):570-9.
- Galli F, Benedetti S, Floridi A, Canestrari F, Piroddi M, Buoncristiani E, et al. Glycoxidation and inflammatory markers in patients on treatment with PMMA-based protein-leaking dialyzers. Kidney international. 2005;67(2):750-9.
- Rascio F, Pontrelli P, Accetturo M, Oranger A, Gigante M, Castellano G, et al. A type I interferon signature characterizes chronic antibody-mediated rejection in kidney transplantation. J Pathol. 2015;237(1):72-84.
- Badalamenti S, Catania A, Lunghi G, Covini G, Bredi E, Brancaccio D, et al. Changes in viremia and circulating interferon-alpha during hemodialysis in hepatitis C virus-positive patients: only coincidental phenomena? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42(1):143-50.
- Barril G, Quiroga JA, Sanz P, Rodrìguez-Salvanés F, Selgas R, Carreño V. Pegylated interferon-alpha2a kinetics during experimental haemodialysis: impact of permeability and pore size of dialysers. Alimentary pharmacology & therapeutics. 2004;20(1):37-44.
- Hofbauer R, Moser D, Frass M, Oberbauer R, Kaye AD, Wagner O, et al. Effect of anticoagulation on blood membrane interactions during hemodialysis. Kidney international. 1999;56(4):1578-83.
- Masakane I, Esashi S, Yoshida A, Chida T, Fujieda H, Ueno Y, et al. A new polymethylmetacrylate membrane improves the membrane adhesion of blood components and clinical efficacy. Renal Replacement Therapy. 2017;3(1):32.
- Pertosa G, Simone S, Soccio M, Marrone D, Gesualdo L, Schena FP, et al. Coagulation cascade activation causes CC chemokine receptor-2 gene expression and mononuclear cell activation in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2005;16(8):2477-86.
- de Vries DK, Lindeman JH, Ringers J, Reinders ME, Rabelink TJ, Schaapherder AF. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(5):1064-70.
- Contin-Bordes C, Lacraz A, Precigout V. Potential role of the soluble form of CD40 in deficient immunological function of dialysis patients: new findings of its amelioration using polymethylmethacrylate (PMMA) membrane. NDT Plus. 2010;3 [Suppl 1]:i20-i7.
- Contin C, Pitard V, Delmas Y, Pelletier N, Defrance T, Moreau JF, et al. Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology. 2003b;110(1):131-40.
- Abe M, Hamano T, Wada A, Nakai S, Masakane I. Effect of dialyzer membrane materials on survival in chronic hemodialysis patients: Results from the annual survey of the Japanese Nationwide Dialysis Registry. PloS one. 2017a;12(9):e0184424.