Toraymyxin® has been used to treat severe unresponsive COVID-19 patients in Europe, Russia, Asia and the U.S.
Toraymyxin® has been approved by US FDA as Investigational Device Exemption (IDE) for the management of COVID-19 patients with septic shock and high level of endotoxemia (EAA™ = 0.6-0.9).
Toraymyxin® has been approved by Health Canada for the management of COVID-19 patients with severe ARF
Polymyxin B Hemoperfusion therapy (Toraymyxin®):
- Direct endotoxin neutralization [1, 2]
- Direct immune cell apheresis [3, 4]
- Restoration of immune balance [5]
- Improvement of lung function [6, 7]
- Rapid recovery of hemodynamics [7, 8]
Pathophysiological aspects in COVID-19 patients
Patients with SARS-CoV-2 infection may present with mild, moderate, or severe illness; the latter includes severe pneumonia, ARF, ARDS, AKI, endothelial damage, coagulopathy, sepsis and septic shock [9-11].
Pneumonia is the most common complication in COVID-19 patients. In severe cases this may be accompanied by a dysregulated immune response. Neutrophils have been found to play an important role as a generator of this response[12] and rollling of neutrophils is one of the key factors in cytokine over-production [13].
Although coronaviruses are known as respiratory pathogens, they clearly have an ability to cross barriers and enter and alter other organ systems [14]. One example of this is the gut-lung axis.
Up to 60% of COVID-19 patients have gastrointestinal symptoms at admission or developed during hospitalization and viral RNA was found to be present in the faeces in 48% of patients [15]. Gastrointestinal dysfunction may lead to increased mucosal permeability and leakage of endotoxin.
Patients who are hospitalized for extended periods in an ICU are more prone to superimposed infections [16]. Gram-negative infection and/or direct mucosal gut translocation lead to the circulation of endotoxin (endotoxemia) [17].
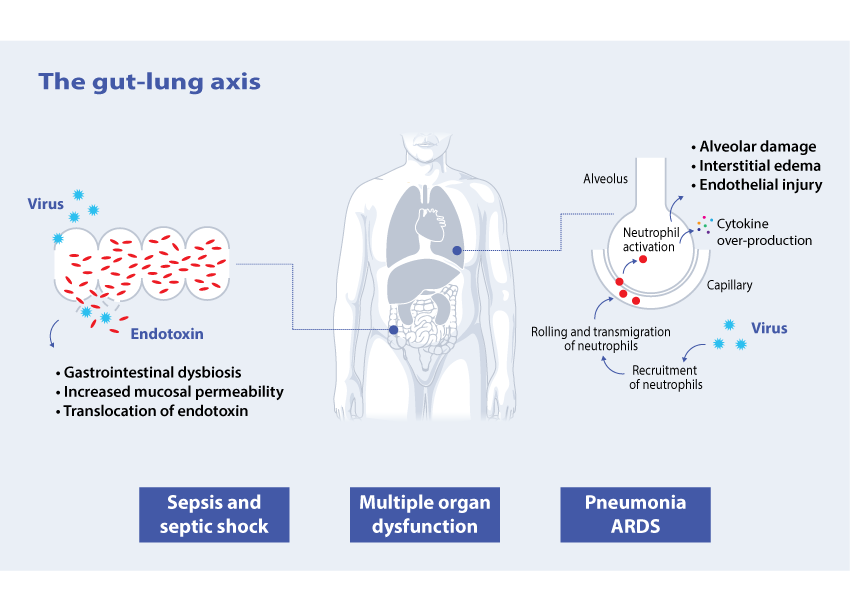
The role of Polymyxin B hemoperfusion in severe COVID-19 patients
Considering the current knowledge of the pathophysiology of COVID-19 and similar viral infections, it is rational to consider a potential role of Polymyxin B Hemoperfusion (medical device Toraymyxin®) as an additional therapy in unresponsive patients affected by COVID-19.
Toraymyxin® has been used successfully to treat patients during other viral pandemics including the avian flu (H5N1) and swine flu (H1N1), which are also characterized by acute respiratory failure [18-20].
In previous publications, the use of Toraymyxin® in patients with viral infection demonstrated improvement in chest radiograph results and lung function, and successful weaning from mechanical ventilation [18-20]. Learn more
The positive effect of PMX-HP in a clinical context of ALI/ARDS characterized by severe alveolar and interstitial edema, a large amount of neutrophil infiltration leading to increased vascular permeability and impaired gas exchange in patients,is well established.
In an animal study using histological samples of rat lung tissue, it was demonstrated that PMX-HP treatment suppresses neutrophil infiltration improving lung microcirculation [21] (See the figure below). This is consistent with further publications demonstrating that PMX-HP selectively removes activated neutrophils [22].
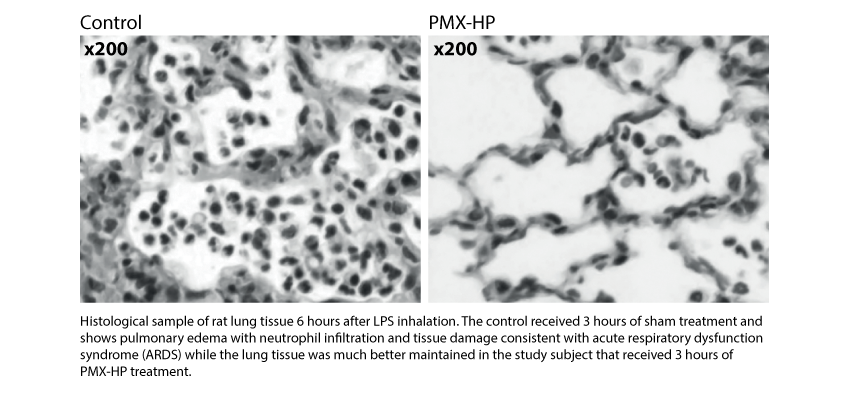
Kushi et al.[6] studied the effect of PMX-HP therapy in 36 septic patients complicated with ARDS. The researchersexamined correlations between IL-8, neutrophil elastase (NE – index of neutrophil activation), PAI-1 (index of vascular endothelial activation) and pulmonary oxygenation (PaO2/FiO2). At one month after PMX-HP therapy all patients werealive, PaO2/FiO2 had significantly improved (244 ± 26.3 to 352 ± 25.4, p < 0.05). IL-8, NE and PAI-1 levels had significantly decreased. Improvement in pulmonary oxygenation appeared to be related to the decrease in neutrophil elastase and IL-8.
In addition, various studies have evaluated the use of PMX-HP in patients with Interstitial Lung Disease.
A pilot study of 16 patients to investigate the mechanism of PMX-HP in idiopathic pulmonary fibrosis with acute exacerbation demonstrated the capacity of PMX-HP to adsorb activated neutrophils [3].
In a subsequent study, 160 patients with interstitial pneumonia received PMX-HP for two consecutive days. PaO2/FiO2 ratio was significantly improved by the end of the second PMX-HP session (173.9 ± 105.4 to 195.2 ± 106.8, p = 0.003) and white blood cell count was significantly decreased (13,330 ± 7,002 to 9,426±5,188/mm3, p < 0.001). 30-day survival rate was 70.1% [23].
References
- Tani, T., et al., Extracorporeal removal of endotoxin: the polymyxin B-immobilized fiber cartridge. Contrib Nephrol, 2010. 167: p. 35-44. (Pubmed)
- Yamashita, C., et al., In Vitro Study of Endotoxin Adsorption by a Polymyxin B-Immobilized Fiber Column. Blood Purif, 2018. 46(4): p. 269-273. (Pubmed)
- Abe, S., et al., Neutrophil adsorption by polymyxin B-immobilized fiber column for acute exacerbation in patients with interstitial pneumonia: a pilot study. Blood Purif, 2010. 29(4): p. 321-6. (Pubmed)
- Nishibori, M., et al., Specific Removal of Monocytes from Peripheral Blood of Septic Patients by Polymyxin B-immobilized Filter Column. Acta Med Okayama, 2009. 63(1): p. 65-9. (Pubmed)
- Esteban, E., et al., Immunomodulation in sepsis: the role of endotoxin removal by polymyxin B-immobilized cartridge. Mediators Inflamm, 2013. 2013: p. 507539. (Pubmed)
- Kushi, H., et al., Early hemoperfusion with an immobilized polymyxin B fiber column eliminates humoral mediators and improves pulmonary oxygenation. Crit Care, 2005a. 9(6): p. R653-61. (Pubmed)
- Klein, D.J., et al., Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med, 2018. (Pubmed)
- Cruz, D.N., et al., Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. Jama, 2009. 301(23): p. 2445-52. (Pubmed)
- Fanelli, V., et al., Acute kidney injury in SARS-CoV-2 infected patients. Crit Care, 2020. 24(1): p. 155. (Pubmed)
- Huang, C., et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020. 395(10223): p. 497-506. (Pubmed)
- Klok, F.A., et al., Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res, 2020. (Pubmed)
- Bordon, J., et al., Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis, 2013. 17(2): p. e76-83. (Pubmed)
- Grommes, J. and O. Soehnlein, Contribution of neutrophils to acute lung injury. Mol Med, 2011. 17(3-4): p. 293-307. (Pubmed)
- Openshaw, P.J., Crossing barriers: infections of the lung and the gut. Mucosal Immunol, 2009. 2(2): p. 100-2. (Pubmed)
- Lin, L., et al., Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut, 2020. 69(6): p. 997-1001. (Pubmed)
- Ronco, C. and T. Reis, Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol, 2020. (Pubmed)
- Fink, M.P. and R.L. Delude, Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin, 2005. 21(2): p. 177-96. (Pubmed)
- Binh, N.G., et al., Polymyxin-B-immobilized-fiber column hemoperfusion with oseltamivir treatment for ARDS due to influenza H1N1/09. Respirol Case Rep, 2015. 3(2): p. 57-60. (Pubmed)
- Kudo, K., et al., Clinical preparedness for severe pneumonia with highly pathogenic avian influenza A (H5N1): experiences with cases in Vietnam. Respir Investig, 2012. 50(4): p. 140-50. (Pubmed)
- Araki, T., H. Ogawa, and A. Nakashima, Endotoxin adsorption therapy for a patient with severe pneumonia resulting from novel influenza A (H1N1) virus infection. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy, 2011. 15(2): p. 207-208. (Pubmed)
- Iba, T., et al., Effect of hemoperfusion using polymyxin B-immobilized fibers on acute lung injury in a rat sepsis model. Int J Med Sci, 2014. 11(3): p. 255-61. (Pubmed)
- Kumagai, T., et al., Apheresis of activated leukocytes with an immobilized polymyxin B filter in patients with septic shock. Shock, 2010. 34(5): p. 461-6. (Pubmed)
- Cruz, D.N., et al., Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. Jama, 2009. 301(23): p. 2445-52. (Pubmed)