New perspectives in sepsis, particularly in septic AKI, and the role of the dialysis membrane were recently presented by Giuseppe Castellano at the national congress of SIN (Italian Society of Nephrology). Exiting new results show the capability of a new PMMA membrane for continuous therapies of inhibiting complement system activation in LPS-induced AKI and lowering the transition from AKI to CKD damage, opening a new scenario in the management of septic AKI.
Download PdfSeptic AKI: from pathophysiology to the dialysis membrane as therapeutic strategy
Pathophysiology of septic AKI
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (1)whereas septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality (1). The immune response is initiated when pattern recognition receptors (PRRs) on the surface of host immune cells recognize pathogen-associated molecular patterns (PAMPs), like lipopolysaccharide (LPS) and danger-associated molecular patterns (DAMPs), which are released in response to inflammatory stress. The dysregulated host response may lead to a life-threatening organ dysfunction and multiple organ dysfunction syndrome (MODS). Endotoxin is the most potent trigger of the septic cascade and has therefore been studied intensively as a therapeutic target. The polymyxin B immobilized cartridge (Toraymyxin®), combines the potent endotoxin neutralizing capabilities of polymyxin B with extracorporeal hemoperfusion. A number of clinical studies have shown that polymyxin B hemoperfusion (PMX-HP) improves organ function and outcome in septic patients. PMX-HP therapy may also reduce sepsis-induced AKI by reducing the activity of proapoptotic circulating factors (2). In the context of AKI and septic AKI, a growing body of evidence indicates that complement activation contributes to its pathogenesis (3). Short-term effects of complement activation include promoting inflammation and coagulation processes (4).
PMMA-based CRRT and the immune response
Prof. Castellano presented results of research conducted in a recent collaborative study aimed at exploring new pathogenic mechanisms in a swine model of LPS-induced AKI. In particular, the researchers wished to investigate the efficacy of PMMA-based continuous hemofiltration (CVVH) in modulating systemic and tissue immune activation in a swine model of LPS-induced AKI. The results showed that CRRT with Polymethylmethacrylate (PMMA), in contrast to Polysulfone (PS) based membrane, led to the recovery of renal function improving oligo-anuria status and hypotension in animals with septic AKI. This resulted in a reduced requirement for catecholamines. Compared to PS, PMMA reduced infiltration of inflammatory cells in the renal parenchyma. CRRT with PMMA reduced acute renal fibrosis and glomerular thrombi. The Researchers further studied how this effect was mediated and found that PMMA-CRRT reduced renal PTX3 deposits and local and complement activation. PMMA-CRRT reduced systemic complement activation, by reducing the activation of both the classical and alternative pathways. PMMA also decreased LBP serum levels in septic-AKI animals. In addition, PMMA decreased sCD40 serum levels. A whole-genome gene expression profile of swine PBMCs showed that LPS activated circulating leukocytes, but in the PMMA group this effect was reduced.
Implications for COVID-19 patients
The results explained above have implications also for COVID-19 patients with AKI development. It is now known, that severe COVID-19 is associated with a dysregulated host response to an infection, much like what happens in sepsis. Furthermore, patients who are hospitalized for extended periods may be susceptible to superimposed infections, increasing the risk of development and progression of sepsis and septic shock with multiple organ failure (5). Up to 25% of critically ill patients with COVID-19 may develop AKI (6). The complement system has been described to be importantly activated in these subjects and suggested as a proper therapeutic intervention target (7).
In a recent article published in Nature Reviews, PMX-HP is recommended in COVID-19 patients with septic shock, with suspected or confirmed Gram-negative bacterial infections and high level of endotoxemia (8). The same group of researchers recently published a flowchart for the use of PMX-HP in septic shock patients (9) suggesting it as first complementary intervention in the described clinical conditions.
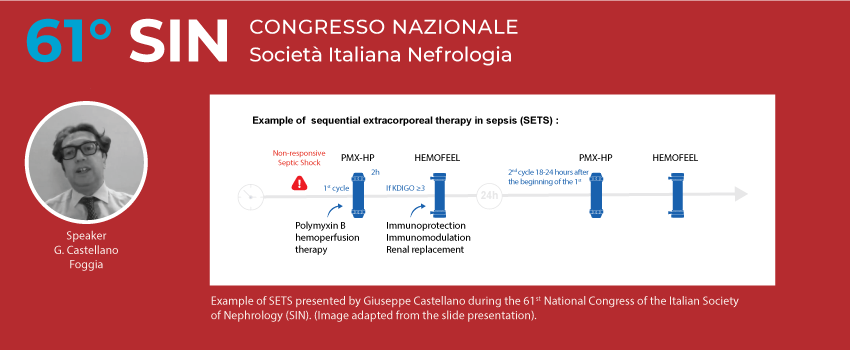
A model for intervention
From the results presented above it is clear that endotoxin (LPS) induces an immune response that can be dysregulated, not only at systemic level, but also specifically at renal level.
PMMA-based CRRT has been shown to attenuate the transition from AKI to CKD in septic AKI. In order to maximize the therapy, the concept of sequential extracorporeal therapy in sepsis (SETS) has been developed. In patients with septic shock who develop AKI with necessity of renal support, sequential therapy, considering PMMA membrane CRRT after PMX-HP seems to be a rational and pragmatic approach. In addition, new technology offered by a specifically designed CRRT machine, guarantees automated management of sequential therapy (SETS) with the combination of the 2 therapy proposals:
- PMX-HP: first intervention achieving the most neutralization of endotoxin and activated immune cells (activated monocytes and neutrophils)
- PMMA-CRRT: second intervention to be followed in case of AKI with necessity of CRRT allowing the maximum hemocompatibility without complement system activation and providing adsorption of pro-inflammatory mediators including cytokines.
The webinar (in Italian) can be watched on-demand by registered participants on the congress website.
References
- Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA : the journal of the American Medical Association. 2016;315(8):801-10.
- Cantaluppi V, Assenzio B, Pasero D, Romanazzi GM, Pacitti A, Lanfranco G, et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive care medicine. 2008;34(9):1638-45.
- Franzin R, Stasi A, Fiorentino M, Stallone G, Cantaluppi V, Gesualdo L, et al. Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage. Frontiers in immunology. 2020;11:734.
- Poppelaars F, Faria B, Gaya da Costa M, Franssen CFM, van Son WJ, Berger SP, et al. The Complement System in Dialysis: A Forgotten Story? Frontiers in immunology. 2018;9:71.
- Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney international. 2020;98(2):314-22.
- Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, et al. Acute kidney injury in SARS-CoV-2 infected patients. Critical care (London, England). 2020;24(1):155.
- Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20(6):343-4.
- Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nature reviews Nephrology. 2020.
- De Rosa S, Villa G, Ronco C. The golden hour of polymyxin B hemoperfusion in endotoxic shock: The basis for sequential extracorporeal therapy in sepsis. Artificial organs. 2019.