In un recente articolo Spotlight pubblicato su The Lancet Respiratory Medicine, Claudio Ronco, Paolo Navalesi e Jean Louis Vincent discutono sul possibile ruolo delle terapie extracorporee di supporto d’organo nel contesto di terapia intensiva durante un’epidemia virale, come l’attuale di COVID-191.
Vengono discussi diversi approcci, tra cui l’emoperfusione, e gli autori concludono:
“Per quanto l’epidemia 2019-nCoV si evolva, il personale di terapia intensiva deve essere preparato ed addestrato per applicare interventi precoci ed ottimali. Le terapie extracorporee di supporto d’organo potrebbero rappresentare una parte importante della risposta ed i medici e gli altri operatori sanitari devono avere familiarità con questa sofisticata terapia. È necessario un invito alla sensibilizzazione sulle diverse tecniche extracorporee, ciascuna con criteri e modalità specifici di prescrizione, somministrazione e monitoraggio”.
ESPERIENZE CLINICHE DI POLYMYXIN B HEMOPERFUSION IN CASI GRAVI DI INFLUENZA VIRALE
L’emoperfusione di Polimixina B è una terapia extracorporea di supporto d’organo ampiamente utilizzata come trattamento complementare nello shock settico. Questa terapia combina le potenti capacità di neutralizzazione dell’endotossina della Polimixina B con l’emoperfusione extracorporea. Il legame ad alta affinità dell’endotossina con la Polimixina B può rimuovere fino al 90% dell’endotossina circolante dopo due trattamenti. Oltre alla neutralizzazione dell’endotossina, sono stati dimostrati altri meccanismi di immunomodulazione. Alcuni di questi derivano dall’eliminazione dell’endotossina, mentre altri dall’adsorbimento diretto di cellule attivate (monociti, neutrofili, linfociti) comportando una riduzione dei mediatori dell’infiammazione, come IL-6, HMGB-1, PAI-1 e altri.
Di seguito riassumiamo le esperienze cliniche ad oggi disponibili in letteratura in merito all’utilizzo della terapia di Polimixina B in emoperfusione in pazienti con infezioni virali (H1N1 – H5N1 influenza) che sviluppano polmonite grave e/o ARDS.
Polymyxin-B-immobilized-fiber column hemoperfusion with oseltamivir treatment for ARDS due to influenza H1N1/09 [2]
This report presents two cases of ARDS due to influenza in Vietnam. Both cases were similar in terms of starting symptoms, the rapid progression to ARDS, and the treatment strategy, PMX-HP therapy and oseltamivir. However, the clinical course of disease and the outcomes were different. For case 1, treatment was initiated on day 4 following the onset of hypoxemia due to ARDS. Symptoms improved rapidly after treatment and the patient was discharged on day 12. For case 2, treatment was initiated on day 9 after the onset of symptoms. Despite intensive therapy, the patient died on day 18. Treatment with PMX-HP and oseltamivir appears to be effective for ARDS due to influenza if initiated early.
Case 1 at ICU admission (A1) and (A2) at discharge after PMX-HP chest radiograph is shown below:
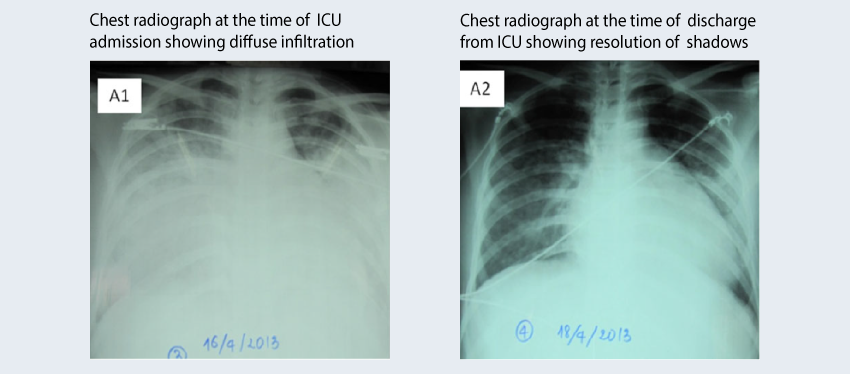
Binh, N.G., et al. Respirol Case Rep, 2015. 3(2): p. 57-60. (Pubmed)
Clinical preparedness for severe pneumonia with highly pathogenic avian influenza A (H5N1): experiences with cases in Vietnam [3]
Treatment of 4 patients with acute respiratory distress syndrome (ARDS) was assessed for renal replacement therapy using continuous hemodiafiltration (CHDF), polymyxin B hemoperfusion (PMX-HP), or their combination. Results: Time to initiation of oseltamivir from symptom onset was 2-6 days for survivors (n=2) and 7-9 days for non-survivors (n=2). All patients except one had infiltrative shadows on chest radiographs on admission. Patients with delayed treatment developed ARDS. Renal replacement therapy contributed to patient survival, with improvement of oxygenation and a dramatic decrease in serum cytokine levels if initiated earlier.
Conclusions: Chest radiography assists early diagnosis and initiation of antiviral treatment. CHDF and PMX-HP are possible candidates for effective treatment of ARDS with H5N1 if applied early.
Kudo, K. et al. Respir Investig, 2012. 50(4): p. 140-50. (Pubmed)
Endotoxin adsorption therapy for a patient with severe pneumonia resulting from novel influenza A (H1N1) virus infection[4]
Case presentation: A 44-year-old man was admitted to the hospital with a one-week history of high fever and increasing dyspnea. A chest CT revealed patchy areas of consolidation and ground-glass opacity. The patient was intubated and mechanically ventilated; piperacillin, ciprofloxacin, methylprednisolone, and sivelestat was started immediately after admission; however, the fever and hypoxemia persisted, and chest radiography revealed progressive consolidation. The patient was diagnosed with severe pneumonia resulting from H1N1 virus infection on the seventh hospital day. Oseltamivir was administered and, in addition, PMX-HP was performed for 4 hrs on the eighth hospital day.
Outcome: Arterial blood gas analysis under mechanical ventilation with 90% oxygen revealed that the partial pressure of oxygen dramatically increased from 65 mmHg to 195 mmHg immediately after PMX-HP therapy. After this therapy, the patient’s clinical condition gradually improved; the patient was weaned from mechanical ventilation on the 16th hospital day. Thereafter, the patient recovered completely and was discharged without complications.
Araki, T. et al. Therapeutic apheresis and dialysis, 2011. 15(2): p. 207-208. (Pubmed)
A Case of Successful Treatment with Polymyxin B-immobilized Fiber Column Direct Hemoperfusion in Acute Respiratory Distress Syndrome after Influenza A Infection [5]
Case presentation: A 56-year-old Japanese man was admitted to the hospital in January 2010 because of progressive dyspnea, hypoxemia, fever and bilateral diffuse infiltration on chest radiograph after pandemic influenza A infection. His chest computed tomography showed diffuse and patchy bilateral ground-glass opacities, and he was diagnosed with ARDS after influenza A infection. The patient was treated with PMX-HP in addition to treatment with oseltamivir, corticosteroid, sivelestat and antibiotics with mechanical ventilation.
Outcome: The patient recovered with only minor pulmonary fibrotic change. Although the efficacy of PMX-HP treatment in patients with ARDS after influenza virus infection is not well established, this treatment could be a possible therapeutic modality in treating the patients with this disease.
Yatera, K. et al. Intern Med, 2011. 50(6): p. 601-5. (Pubmed)
Hypercytokinemia with 2009 pandemic H1N1 (pH1N1) influenza successfully treated with polymyxin B-immobilized fiber column hemoperfusion [6]
Case presentation: 16-year-old female with no medical history developed fever, general fatigue, and diarrhea. She was diagnosed with type A influenza.
Outcome: PMX hemoperfusion reduced the elevated inflammatory cytokine levels and particularly the high-mobility group box (HMGB)-1 level, suggesting that suppression of inflammation in the lungs improved oxygenation. In severe cases of respiratory distress, PMX-HP may offer a treatment method.
Takeda, S. et al. Intensive Care Med, 2010. 36(5): p. 906-7. (Pubmed)
A case of severe ARDS caused by novel swine-origin influenza (A/H1N1pdm) virus: a successful treatment with direct hemoperfusion with polymyxin B-immobilized fiber [7]
Case presentation: A 35-year-old female with Down syndrome was admitted to the hospital because of severe pneumonia caused by an infection with the novel swine-origin influenza (A/H1N1pdm) virus (S-OIV). A chest X-ray on admission revealed bilateral infiltration shadows. Although mechanical ventilation was administered because of the development of ARDS, the hypoxemia continued to progress. Evidence of alveolar hemorrhage was observed on evaluation of the patient using bronchofiberscopy. Bacterial examination was negative. Despite intensive care, including respiratory management, the patient’s hypoxemia and hypotension progressed. It was suspected that hypercytokinemia due to the influenza infection resulted in septic shock. The patient was subsequently treated with PMX-HP.
Outcome: Hypoxemia improved immediately upon PMX-HP treatment. The patient was free from mechanical ventilation and discharged from the hospital by the 17th day of hospitalization. PMX-HP seems to improve hypoxemia in patients with severe ARDS who cannot maintain sufficient respiratory control under mechanical ventilation.
Yokoyama, T. et al., J Clin Apher, 2010. 25(6): p. 350-3. (Pubmed)
References
- Ronco, C., P. Navalesi, and J.L. Vincent, Coronavirus epidemic: preparing for extracorporeal organ support in intensive care.The Lancet. Respiratory medicine, 2020: p. S2213-2600(20)30060-6. (Pubmed)
- Binh, N.G., et al., Polymyxin-B-immobilized-fiber column hemoperfusion with oseltamivir treatment for ARDS due to influenza H1N1/09. Respirol Case Rep, 2015. 3(2): p. 57-60. (Pubmed)
- Kudo, K., et al., Clinical preparedness for severe pneumonia with highly pathogenic avian influenza A (H5N1): experiences with cases in Vietnam. Respir Investig, 2012. 50(4): p. 140-50. (Pubmed)
- Araki, T., H. Ogawa, and A. Nakashima, Endotoxin adsorption therapy for a patient with severe pneumonia resulting from novel influenza A (H1N1) virus infection. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy, 2011. 15(2): p. 207-208. (Pubmed)
- Yatera, K., et al., A case of successful treatment with polymyxin B-immobilized fiber column direct hemoperfusion in acute respiratory distress syndrome after influenza A infection. Intern Med, 2011. 50(6): p. 601-5. (Pubmed)
- Takeda, S., et al., Hypercytokinemia with 2009 pandemic H1N1 (pH1N1) influenza successfully treated with polymyxin B-immobilized fiber column hemoperfusion. Intensive Care Med, 2010. 36(5): p. 906-7. (Pubmed)
- Yokoyama, T., et al., A case of severe ARDS caused by novel swine-origin influenza (A/H1N1pdm) virus: a successful treatment with direct hemoperfusion with polymyxin B-immobilized fiber. J Clin Apher, 2010. 25(6): p. 350-3. (Pubmed)